-
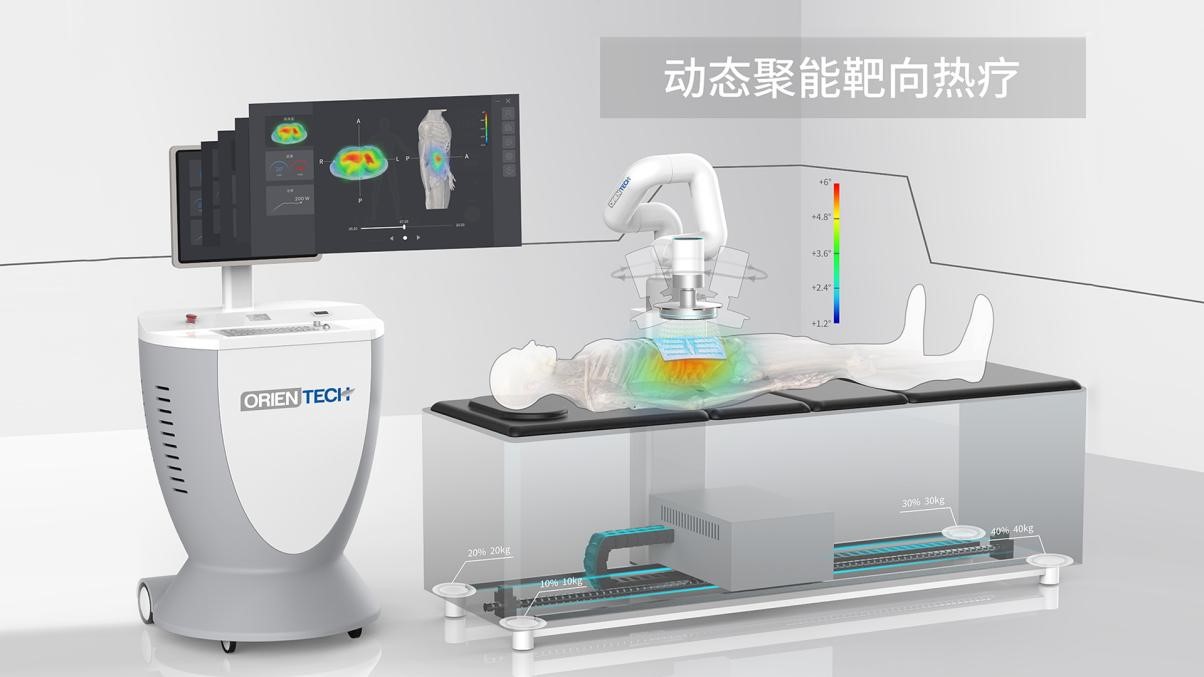
BSD热疗设备 +
BSD技术参数: 四通道输出 频率75MHz-120MHz可调,具备相位,振幅调整和入射、反射功率、相位校正功能......
Read More
-
德国品质cymedics医学美容设备 +
我们摒弃对人体有害的化妆品或美容整形手术,选择使用生物控制论程序,通过先进的电脑软件系统,激活人体自身的再生过程。
Read More
-
关于大连奥瑞科技有限公司 +
大连奥瑞科技有限公司 奥瑞科技是中国领先的医疗科技公司,是向全国范围内提供医疗产品和医疗项目的最具规模的综合性服务供应商之一。
Read More
Indications
- Lesion less than 6cm depth subcutaneously. For example: lymphoma, breast cancer, mammitis, breast lobule hyperplasia, rectal cancer, prostate cancer, prostatitis and prostatic hyperplasia, esophageal cancer, cervical cancer, ovarian and ovarian appendage lesion, arthropathy, etc.
Contraindications
- Patients with pacemaker cannot receive treatment or stay near to the device.
- Severe cardiovascular and cerebrovascular diseases
- Epilepsy
- Fever, cachexia or people with tendency of massive hemorrhage
- Sensitive sites such as eyeballs and testis
- Metal materials in focal region
- Brain Region
- Heart area of patients with hypertension and heart disease
- Abdomen of pregnant patients
- Nervous damage poor thermal sensitivity in treating area